Choosing a label that is made from pharmaceutical grade materials is crucial to ensuring that pharmaceutical products are safe. At UPM Raflatac, we assure the safety of our labels by using materials that have been rigorously pre-tested, meet applicable regulatory requirements for their intended use and offer a secured long-term supply.
Pharmaceutical labels developed, formulated and manufactured for regulatory compliance
Protecting people
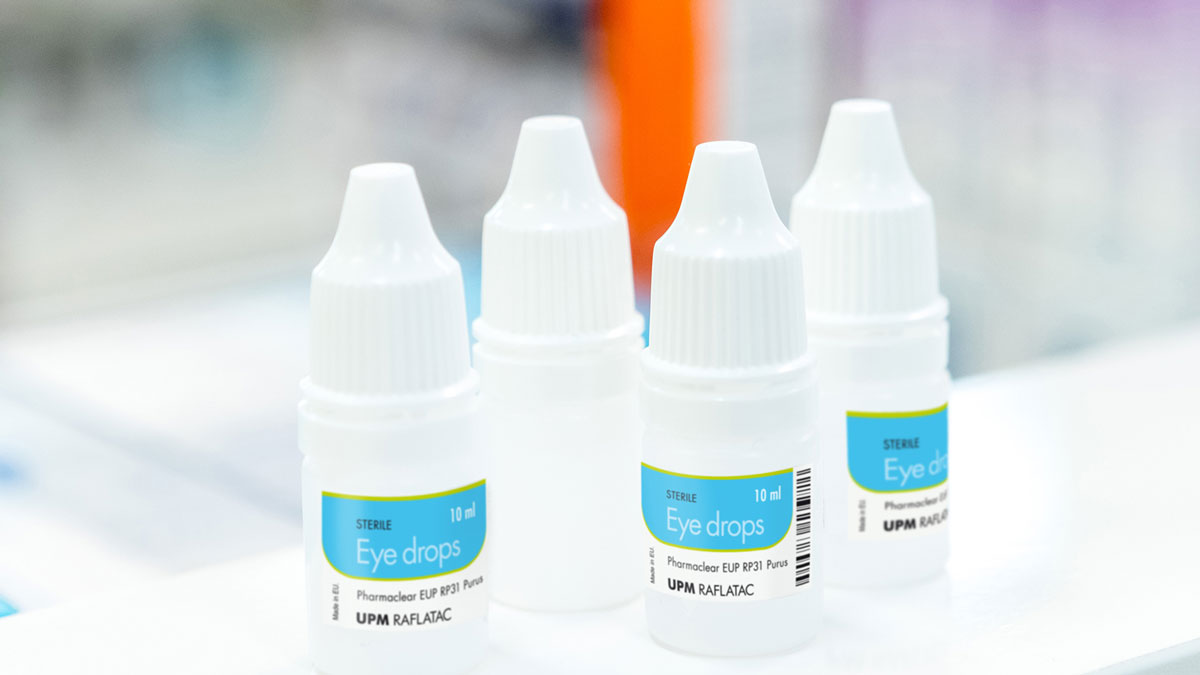
Low migration pharmaceutical labels
Labels play a role in protecting safety by carrying crucial medical information. Labels can also cause concern in cases when certain pharmaceutical products require low migration materials. Examples of pharmaceutical products that may require low leachable labels are liquid drugs packed in plastic packaging, such as eye drops, nasal sprays, or injectables. Our selected pharmaceutical materials and advanced adhesive technology are developed in accordance with industry guidelines, such as:
- EMA (European Medicines Agency) /CVMP/205/04 Guideline on Plastic Immediate Packaging Materials (2005)
- FDA (Food and Drug Administration, USA) Guidance on Container Closure Systems for Packaging Human Drugs and Biologics (1999)
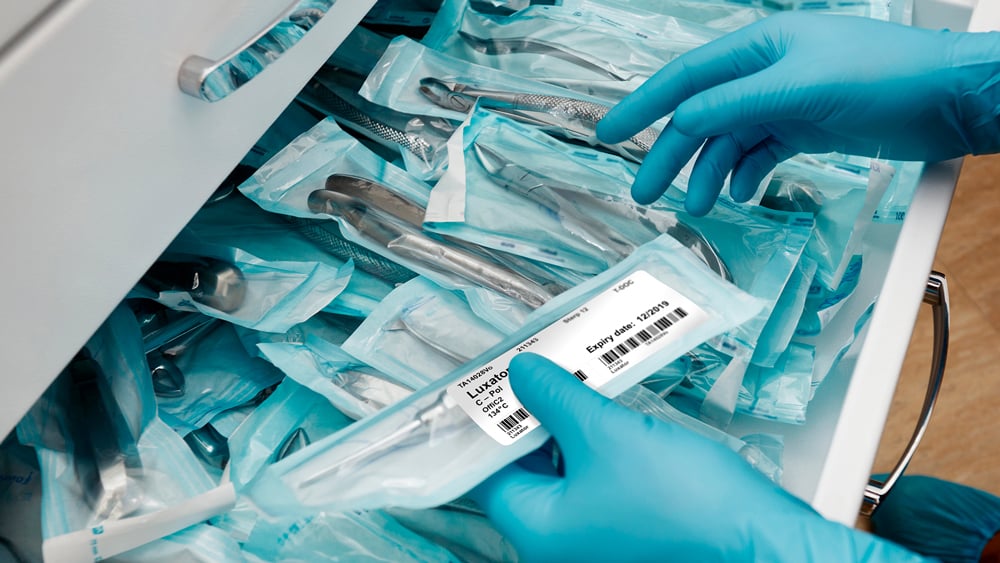
Medical device regulations
Medical device guidelines require that the printing and labeling systems are compatible with the sterile barrier system processing, device and materials as well as remain intact and legible until the point of use. Medical device labeling helps patients or end-users in understanding the device, its operation, care, and maintenance. Medical device regulations include:
- The new emerging global Medical Device regulation includes the adoption of a Unique Device Identification (UDI) serialization system to mark and identify medical devices in the healthcare supply chain. One of the latest UDI regulatory updates is the new EU Medical Device Regulation [MDR], with a May 2020 implementation deadline, which is a similar system to the one adopted by the US FDA in 2013.
- The In Vitro Diagnostics Regulations (IVDR) is the regulatory basis for placing on the market, making available and putting into service in vitro diagnostic medical devices on the European market. The deadline for implementing the In Vitro Diagnostics Regulations (IVDR) in EU is May 2022.
Falsified Medicines Directive (2011/62/EU)
The Falsified Medicines Directive (2011/62/EU) regards packaging for prescription drugs and high-risk, over-the-counter medicines. Compliance is achieved by incorporating tamper-evident features as described in ISO 21976 standard ‘Packaging — Tamper verification features for medicinal product packaging’, supporting the harmonization and implementation of tamper verification features to the packaging of medicinal products worldwide.
Packaging regulations
Our label materials for pharmaceuticals and healthcare are designed to meet packaging regulations. Our label materials comply with all relevant applicable regulatory and safety requirements for packaging and food contact for their intended use:
- Packaging regulations concerning heavy metals regarding EU Directive 94/62/EC and the Toxics in Packaging Clearinghouse (TPCH) formerly known as the Coalition of North-eastern Governors’ (CONEG, US).
- Adhesives have been assessed for European food-contact regulations;
- Food Framework Regulation (EC) No 1935/2004 on materials and articles intended to come into contact with food and
- Regulation (EC) No 2023/2006 on good manufacturing practice for materials and articles intended to come into contact with food.
- FDA Food Contact Compliance:
- FDA 21 CFR § 110 – Good Manufacturing Practices (GMP)
Why use dedicated pharmaceutical labeling solutions?
Labels carry crucial medical information throughout the life cycle of the product, while also playing a role in protecting and sealing the contents of the product. High durability, print quality, performance, regulatory compliance and product safety are just some of the features the label must possess. By choosing dedicated pharmaceutical labeling solutions, brands and businesses ensure that those label materials are:
- designed and pre-tested for pharmaceutical applications
- developed with product safety and compliance in mind
- assured through long term availability and stability, with change management notifications and secured availability
- supported by a documentation service to assist end-user product regulatory validation