It is very complex for pharmaceutical companies to test and validate label materials for their vaccines. One of the most crucial features in label solutions is to ensure their print performance. The right label material and print will remain securely intact and legible after harsh storage and handling conditions, without absorbing moisture or being sensitive for scuffing. Due to the pre-testing collaboration between HP Indigo and UPM Raflatac, it is now easier for pharmaceutical brand owners to select the right vaccine labels and printing inks for their own final testing, saving their time and resources. The new solution concept ensures high performance and stability of the product combination in harsh conditions.
TAKEAWAYS
- HP Indigo, a global pioneer in digital printing, wanted to answer the need of pharmaceutical brand owners for a high-performing printed vaccine labeling solution that could withstand harsh application-specific conditions and very low temperatures (down to -80°C), also ensuring product security.
- HP Indigo collaborated with UPM Raflatac to pre-test different material combinations of labels, inks and varnishes for the demanding application.
- As a result, a high-performing combination was found. The vaccine labels that can withstand the special conditions are printed on an HP Indigo 6K Digital Press using variable data technology powered by HP SmartStream Designer. The compatible label material in this solution is Raflex Plus White TC / RP80 LT / HD70.
- HP Indigo and UPM Raflatac invite pharma brand owners to try this well tested label solution with their products.
Wanted: a reliable printed vaccine labeling solution for harsh and freezing conditions
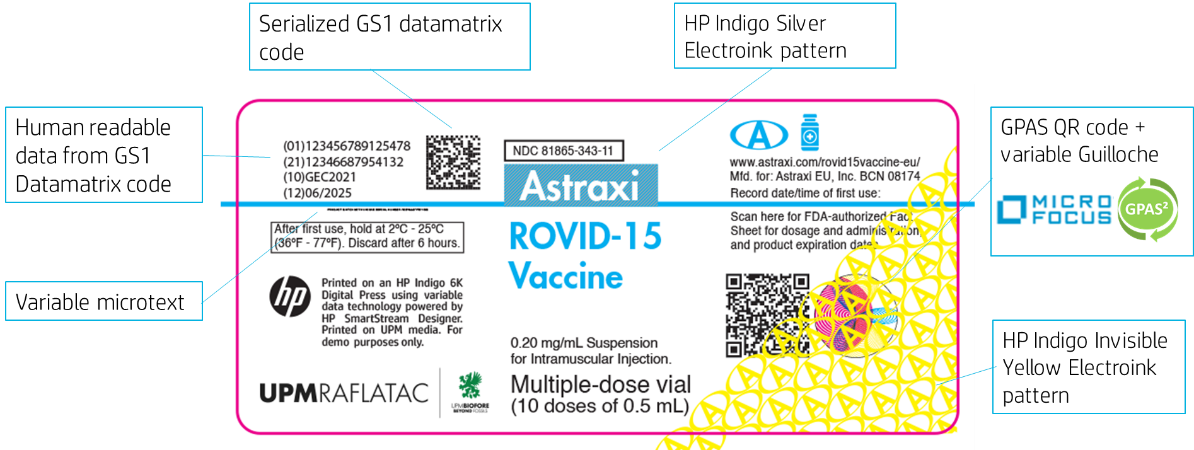
Vaccine label artwork and features
HP Indigo, a world-leader in digital printing, provides smart and secure labels and packaging digitally printed for a variety of applications. Last year during increasing global needs caused by the Covid-19 pandemic, set out to create a digitally printed vaccine labeling solution.
Vaccine labels must withstand harsh conditions – down up to -80°C in storage – during the transportation and distribution process. The labels and their ink must withstand when the vaccines are transferred into ultra-cold freezers at health clinics until final use. The reliability of the materials and the speed of delivery are of extreme importance in this application. Any possibility of label failure must be minimized as it can lead to wasting a dosage of vaccine.
HP Indigo inquired UPM Raflatac for suitable label materials and soon received a variety of samples in their demo center in Barcelona, Spain.
Stress tests to find the right material combination
Following digital printing and overprint varnish process, HP Indigo R&D examined the durability of the ink after exposure to freezing conditions such as a temperature of -80°C. After refrigerating the company performed rubbing, peeling and abrasion tests at labs using standard protocol on how the printed vaccine label works in terms of scuff resistance and water resistance.
To ensure product brand protection the vaccine label was designed with 4 colors and several security elements. First, a sterilized QR code supported by HP Indigo serialization collaboration with MICRO FOCUS GPAS, which delivers brand protection, supply chain traceability, consumer engagement, and sustainability services through a versatile platform customisable to an organisation's exact business requirements. The label also has HP Indigo ElectroInk Invisible yellow for covert layer of authenticity and a serialized micro text and serialized GS1 data matrix code.
The aim of the pre-testing is to help pharma brands to select the right End-to-end application solution for their vaccines from the beginning. Pre-testing saves their time and resources by ensuring that the performance of the product combination meets the requirements of their intended use before starting their own testing activities.
“It is very complex for pharmaceutical companies to test and validate label materials and their most crucial feature – their print performance. This solution concept that we have created together will ease their work. We have pre-screened the best options in terms of the inks, varnishes and label materials for these conditions. As we have these application-specific test protocols and settings made, we can make robust recommendations for pharma prints and brand owners,” says Lottie Andersson, Development Manager, Pharma, UPM Raflatac.
With this well tested label solution, HP Indigo encourages pharma companies to reach out and test it on their products. HP Indigo and UPM Raflatac are happy to guide companies through the process.
“We have a strong global partnership between HP Indigo and UPM Raflatac. Our collaboration takes place on many levels, including technology development, end-use understanding, and sustainability. Together we can help our mutual customers to make the best possible choices for their business,“ says Kirit Naik, Global Director, Digital Printing Technologies at UPM Raflatac.